Pipeline & Clinical Activities
Our Pipeline
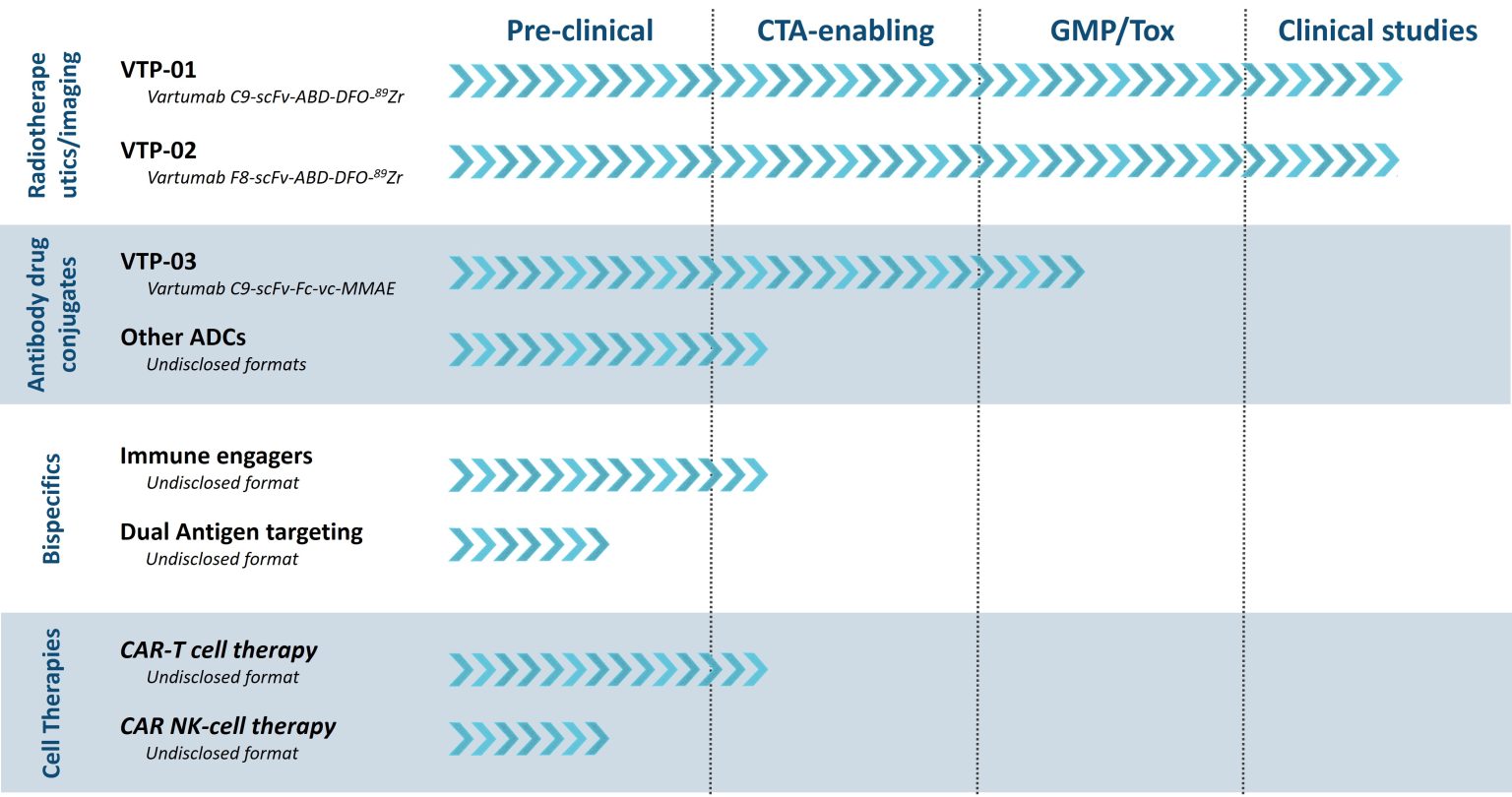
First-in-class therapies against ofCS
We have an extensive pre-clinical data package on the efficacy and safety of Vartumabs for cancer treatment which includes:
- Intravenous injection of Vartumabs into different rodent models results in tumor localization with little to no accumulation in healthy tissues.
- ADC formulations of Vartumabs using different drug payloads show curative effects in xenograft, allograft and patient-derived mice models of various cancers with as little as a single dose of 2 mg/kg DAR2.
- Repeated high dose IV injections (up to 5 mg/kg) show no weight loss or change in blood and enzymatic panels of healthy and various cancer mice models.
- Bispecific T-cell engagers and CAR-Ts have a similar efficacy and safety profile in mouse models.
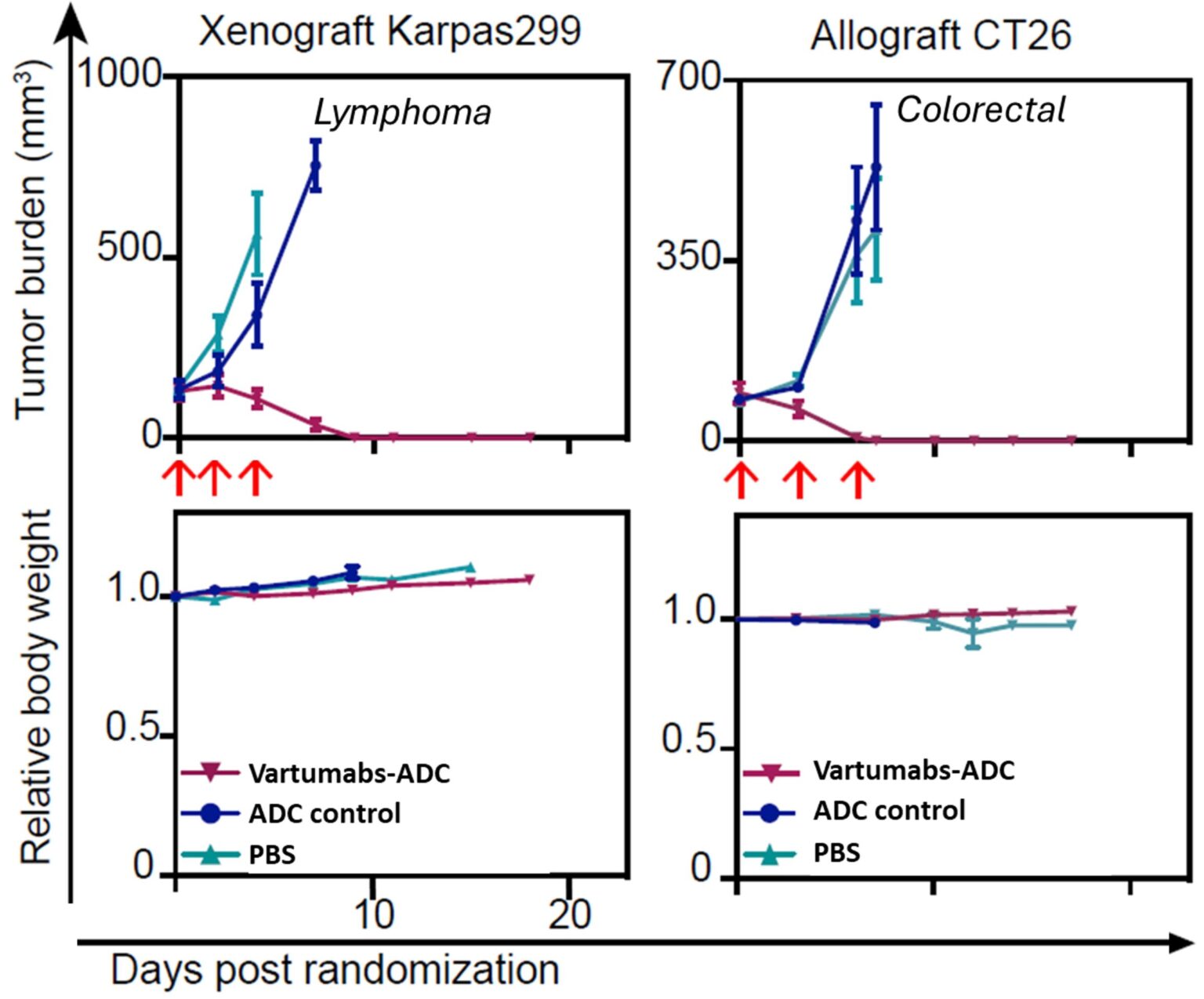
Response of lymphoma and colorectal cancer mice models to treatment with 3 doses of Vartumabs-ADC showing tumor regression and no change in body weight
Clinical Activities
VARTUTRACE (NCT06645808): A first-in-human immune PET/CT Phase 0 microdosing imaging trial started in Dec 2024. The goal of VARTUTRACE is to evaluate the safety, biodistribution, and tumor targeting of two Zirconium89-labeled Vartumabs (VTP-01 and VTP-02) in 32 cancer patients. Preliminary results show highly specific tumor accumulation of VTP-01 in all five patients so far (two with non-small cell lung cancer, one with a rectal cancer and two with an esophageal carcinoma). We are observing minimal off-tumor binding broadly reflecting pre-clinical findings. Critically, we have done no patient stratification, dose optimization or used complex imaging protocols (e.g. injection of cold mAb) to reduce off-tumor binding and increase contrast).
Upcoming Phase I/IIA trial with an antibody drug conjugate: GMP production of VTP-03 initiated in July 2025. VTP-03 is an antibody drug conjugate with a scFv-Fc format engineered to achieve an homogenous DAR2 with vc-MMAE. We anticipate entering the clinic with VTP-03 in 2027.
Our Pipeline: Pushing forward a first-in-class approach
We have obtained solid evidence of the potential efficacy and safety of Vartumabs as a cancer targeting platform. Intravenous injection of Vartumabs into animal models demonstrates high tumor specificity with no apparent off target effects. This indicative safety is supported by repeat high dose IV injections of antibody drug conjugates showing no weight loss, change in blood panels or other adverse effects in several cancers and models, including in xenograft human lymphoma (Karpas), allograft murine colorectal carcinoma (CT26), and patient derived (PDX) Pancreatic (PDAC) and sarcoma mice models. Anti-CD3 fusions have a similar efficacy and safety profile in mouse models, with a dramatic effect on tumor growth and results in either complete stop of tumor growth or full tumor regression depending on the model.

Tumor burden and relative body weight evolution in mice cancer models for Lymphoma (Karpas 299) and murine colorectal carcinoma (CT26)
Phase 0: A First-in-Human Microdosing Study
Our first clinical study will start in Q3 2024, and for which we already achieved scale-up production milestones. This first trial will take the shape of nuclear imaging, first-in-human study with ~ 30 patients in a basket trial format. We expect this study to provide us with the pharmacokinetics and biodistribution of Vartumabs in patients with cancer, paving the way for efficacy trials.

